Events Calendar
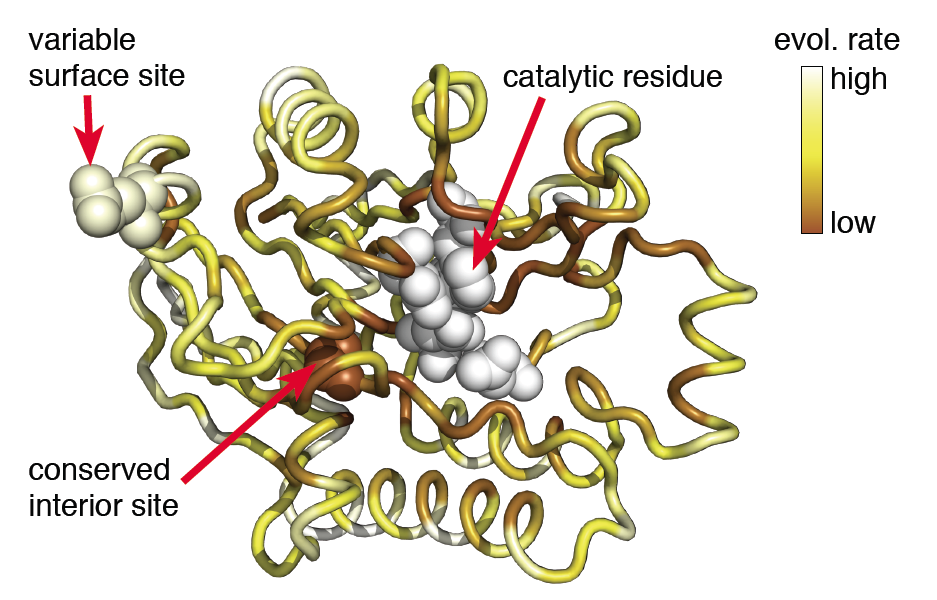
Proteins evolve under constraints determined by their structural and functional properties. These constraints are visible in co-variation among sites and in increase sequence conversation at sties in the protein core, near active sites in enzymes, or at sties involved in protein-protein interfaces. I will discuss first the relative importance of these structural and functional constraints on sequence conservation. Then, I will discuss how co-variation among sites can be used to infer a protein’s contact matrix, and how the accuracy of the inference depends critically on the definition of a medium-residue contact. Finally, I will discuss how, in the context of duplicated genes, selective constraints can percolate through protein-protein interaction networks, such that a duplicated gene imposes significant selection pressure on its non-duplicated partner.