Events Calendar
Professor, Group leader, Biophysics
AMOLF
Amsterdam, The Netherlands

Chaperone-guided folding of single proteins
It is well known that chaperones are central to folding proteins in cells. However, it has been difficult to determine whether chaperones alter folding pathways directly, or instead act indirectly by suppressing aggregation between protein. Moreover, the fundamental mechanisms that chaperones could exploit to productively alter folding pathways have remained obscured. My lab has developed approaches to study this issue using optical tweezers. We have previously studied the chaperones SecB, trigger factor, and Hsp70, which has revealed surprising capabilities to bind and stabilize partially folded structures - and thus act throughout the folding pathways. In this presentation, I will discuss some of these earlier findings as well as more recent results on other chaperone systems, including GroEL and the disaggregase ClpB, which is capable of reversing aggregation reactions.
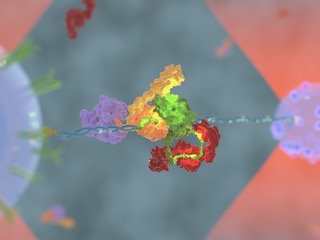
Alternative modes of client binding enable functional plasticity of Hsp70, A. Mashaghi, S. Bezrukavnikov, D.P. Minde, A.S. Wentink, R. Kityk, B. Zachmann-Brand, M.P. Mayer, G. Kramer, B. Bukau and S.J. Tans, Nature 539, 448–451 (2016).
Reshaping of the conformational search of a protein by the chaperone trigger factor, Mashaghi A, Kramer G, Bechtluft P, Zachmann-Brand B, Driessen AJ, Bukau B, Tans SJ, Nature 500, 98-101 (2013).
Direct observation of chaperone-induced changes in a protein folding pathway, Philip Bechtluft, Ruud van Leeuwen , Matthew Tyreman, Danuta Tomkiewicz, Nico Nouwen, Harald Tepper, Arnold Driessen, and Sander J. Tans, Science 318, 1458-61 (2007).


